Defining genetic pathways of myeloid disease progression
In myeloid malignancies, somatic mutations do not arise in random order or in random combinations. Instead, individual mutations can have highly stereotyped positions in the clonal hierarchy and strong patterns of co-mutation association and exclusivity. For example, mutations affecting genes that encode epigenetic modifiers (DNMT3A, TET2, ASXL1, EH2, etc.) or RNA spliceosome components (SF3B1, SRSF2, U2AF1) tend to arise in disease and rarely occur at the time of transformation. By contrast, mutations that drive activated growth factor signaling pathways (NRAS, KRAS, PTPN11, FLT3, etc.) are rarely identified early in disease and instead are typically gained with disease progression. We combine human genetics and laboratory models (mouse and cell lines) to study the mechanisms by which mutations cooperate to drive disease progression, with a focus on the BCOR/PRC1.1 complex.
BCOR/PRC1.1
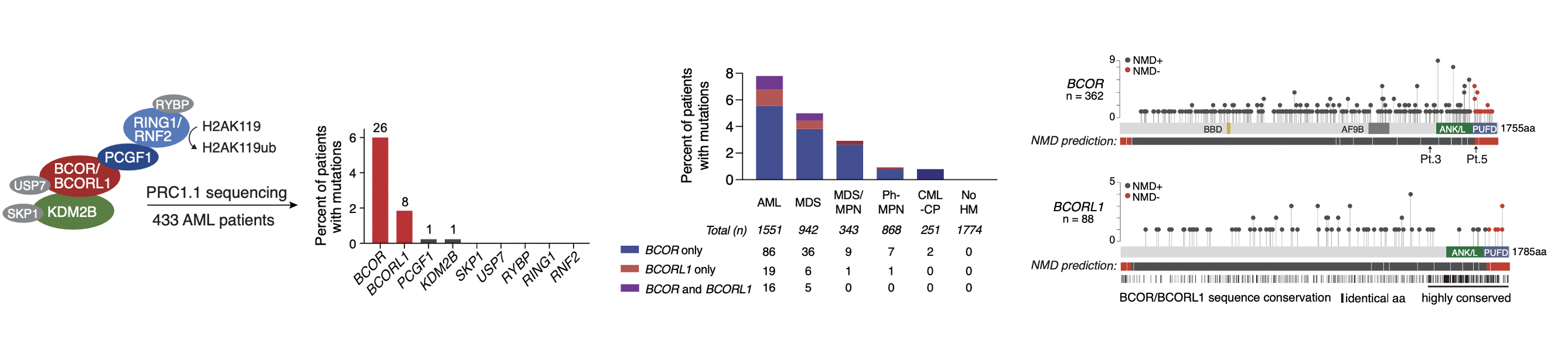
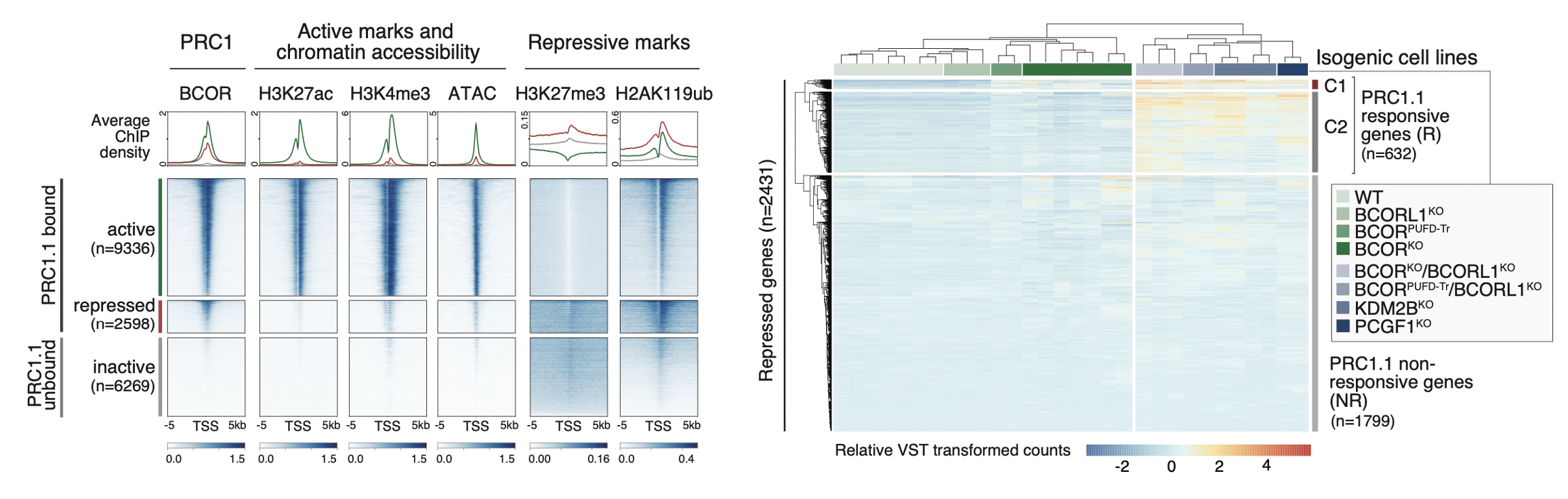
BCOR and its paralog BCORL1 encode subunits of the Polycomb repressive complex 1.1 (PRC1.1) and are recurrently mutated in myeloid malignancies. We have shown that leukemia-associated BCOR/BCORL1 mutations unlink the PRC1.1 RING-PCGF enzymatic core from the KDM2B-containing chromatin targeting auxiliary subcomplex, either by causing complete protein loss or expression of a C-terminally truncated protein lacking the PCGF Ub-like fold discriminator (PUFD) domain. By uncoupling PRC1.1 repressive function from target genes, BCOR/BCORL1 mutations activate aberrant cell signaling programs that confer acquired resistance to tyrosine kinase inhibition. Read more about about our recent BCOR/PRC1.1 study here.
BCOR and its paralog BCORL1 encode subunits of the Polycomb repressive complex 1.1 (PRC1.1) and are recurrently mutated in myeloid malignancies. We have shown that leukemia-associated BCOR/BCORL1 mutations unlink the PRC1.1 RING-PCGF enzymatic core from the KDM2B-containing chromatin targeting auxiliary subcomplex, either by causing complete protein loss or expression of a C-terminally truncated protein lacking the PCGF Ub-like fold discriminator (PUFD) domain. By uncoupling PRC1.1 repressive function from target genes, BCOR/BCORL1 mutations activate aberrant cell signaling programs that confer acquired resistance to tyrosine kinase inhibition. Read more about about our recent BCOR/PRC1.1 study here.