Improving Outcomes after Stem Cell Transplantation
Allogeneic stem cell transplantation involves the transfer of healthy donor cells, including hematopoietic stem cells and mature immune effector cells, to recipients with high-risk hematologic malignancies. While transplantation can be curative for some patients, disease relapse and transplant toxicity remain central clinical challenges. We investigate characteristics of the patient, the disease, and the stem cell donor in order to develop enhanced prognostic models and identify opportunities to improve patient outcomes
Genetic determinants of outcome in patients with myeloid malignancies
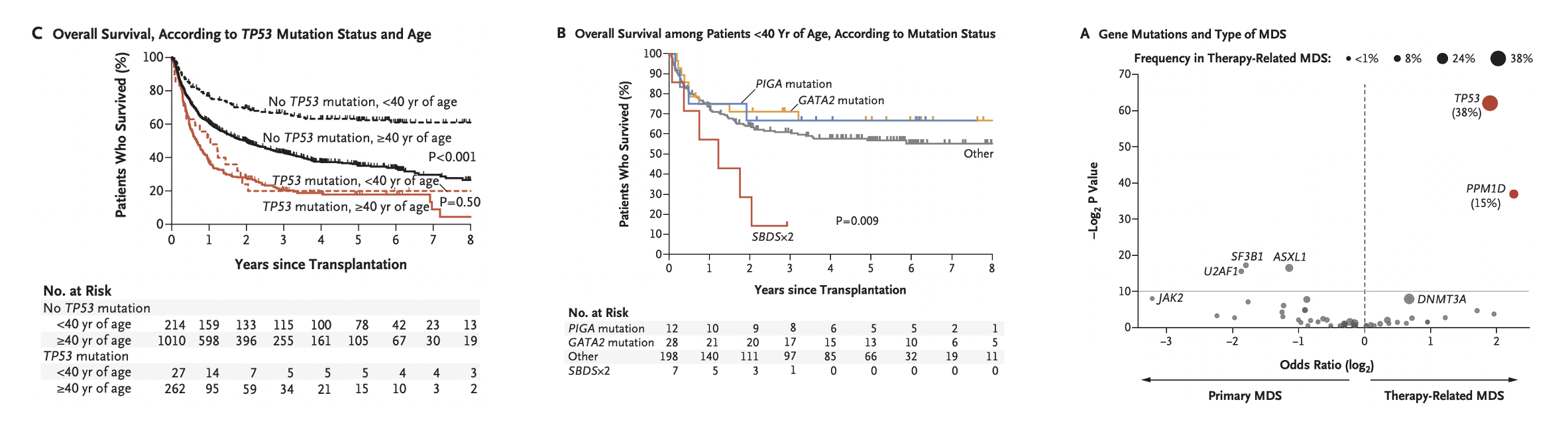
Gene mutations drive the pathogenesis of myeloid malignancies, such as AML, MDS, and MPN, and are closely associated with clinical characteristics. Using genomic approaches, we study patients who have undergone transplantation, and define groups with distinct disease biology and clinical outcomes. These studies identify patients who benefit from standard transplant approaches as well as those who may benefit from investigational approaches. Read about our study here.
Gene mutations drive the pathogenesis of myeloid malignancies, such as AML, MDS, and MPN, and are closely associated with clinical characteristics. Using genomic approaches, we study patients who have undergone transplantation, and define groups with distinct disease biology and clinical outcomes. These studies identify patients who benefit from standard transplant approaches as well as those who may benefit from investigational approaches. Read about our study here.
Telomere maintenance and non-relapse mortality
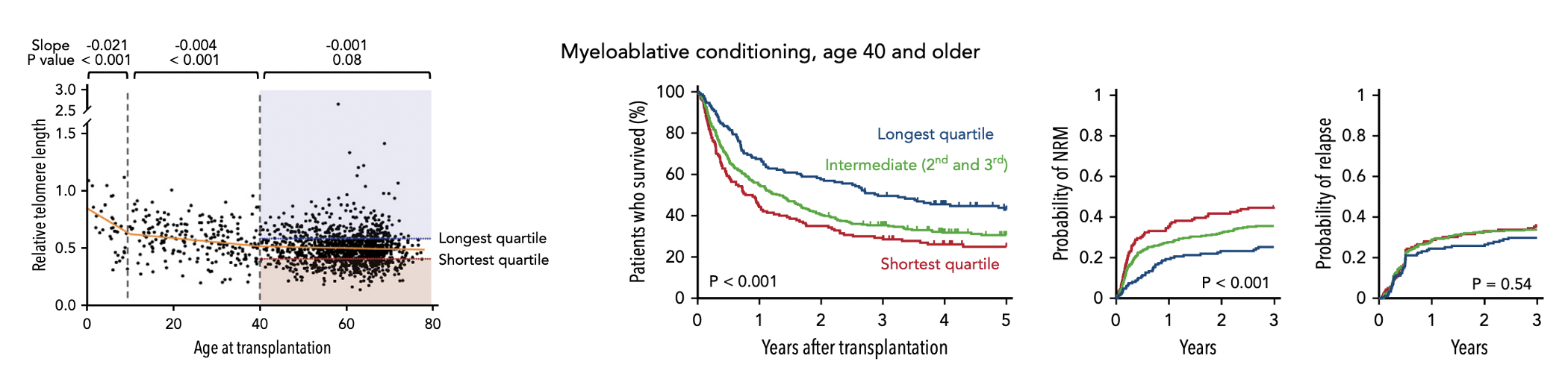
Patient characteristics, such as age and health status, can influence transplant outcomes. We found that shorter recipient telomere length and germline telomerase complex mutations are strong predictors of non-relapse mortality, independent of clinical factors such as age, comorbidities, and disease genetics. We use mouse models and primary human samples to study the mechanisms by which short telomere length may restrict tissue regenerative capacity in the setting of genotoxic or replicative stress after transplantation. Read about this work here.
Patient characteristics, such as age and health status, can influence transplant outcomes. We found that shorter recipient telomere length and germline telomerase complex mutations are strong predictors of non-relapse mortality, independent of clinical factors such as age, comorbidities, and disease genetics. We use mouse models and primary human samples to study the mechanisms by which short telomere length may restrict tissue regenerative capacity in the setting of genotoxic or replicative stress after transplantation. Read about this work here.
Donor clonal hematopoiesis (CH)
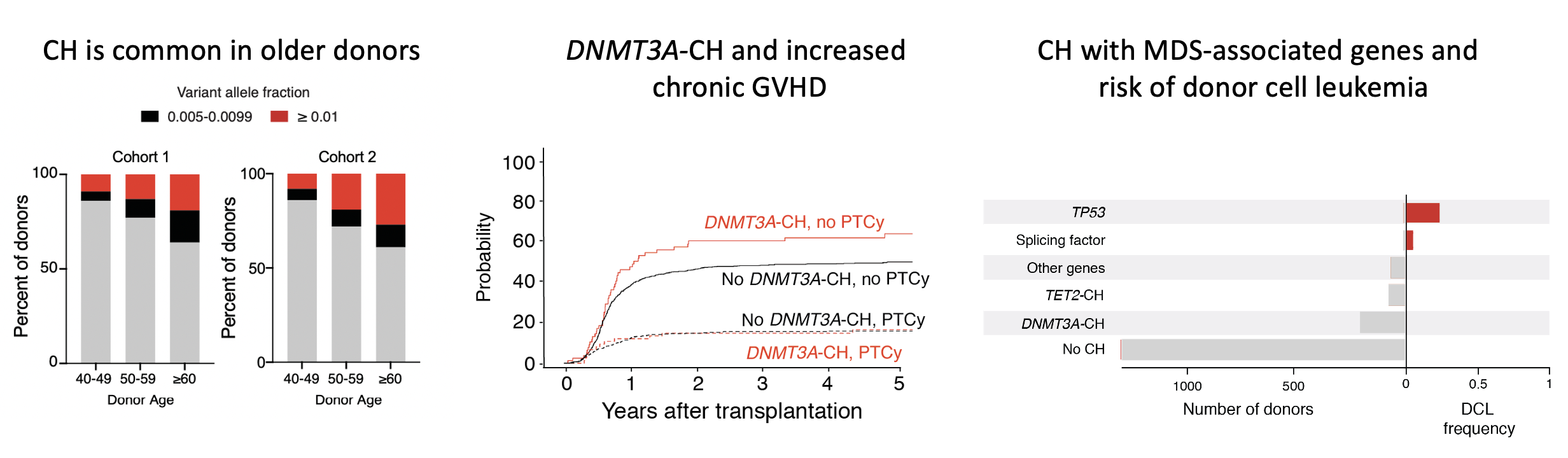
By studying recipients with altered graft function, we found that some healthy stem cell donors have clonal hematopoiesis, marked by mutations affecting genes such as DNMT3A and TET2. These donor clones have the ability to engraft in recipients, undergo selective expansion, and disrupt normal hematopoietic function. We are using mouse GVHD models and primary human samples to investigate the mechanisms by which donor-engrafted clonal hematopoiesis impacts transplantation outcomes. Should donors be screened for CH? Read more about our thoughts on this debate here, and our latest research study here.
By studying recipients with altered graft function, we found that some healthy stem cell donors have clonal hematopoiesis, marked by mutations affecting genes such as DNMT3A and TET2. These donor clones have the ability to engraft in recipients, undergo selective expansion, and disrupt normal hematopoietic function. We are using mouse GVHD models and primary human samples to investigate the mechanisms by which donor-engrafted clonal hematopoiesis impacts transplantation outcomes. Should donors be screened for CH? Read more about our thoughts on this debate here, and our latest research study here.